2014
Technology &
ZetrOZ, Inc.

ZetrOz Astronaut commanders Leroy Chiao (front left) and Ken Bowersox
(front right) try out the ZetrOZ sustained acoustic medicine (SAM)
device
ZetrOZ has developed an ultrasound device, SAM®, Sustained Acoustic Medicine, that enables self-administered therapeutic ultrasound treatment for periods up to four hours outside a physician’s office. Astronauts gain as much as two inches in height in space due to spinal decompression the microgravity environment.
As the discs in the spine expand, the surrounding tissue is stressed and there is irritation of the spinal nerves resulting in back pain. Upon returning to Earth’s gravity, astronauts experience pain as the spine re-adapts to the compression of a 1G environment. Astronauts are 4 times more likely than the general population to develop Herniated Disc Pulposes (HNP) upon their return to Earth. With the funds awarded by NSBRI and matching funds from the Small Business Innovation team at Connecticut Innovations, this breakthrough device will undergo clinical testing to determine its ability to relieve back pain associated with herniated discs.
UPDATE: Where is ZetrOz Now?

sam® Sport is the first and only FDA-cleared wearable device for multi-hour continuous ultrasound therapy.
September 17, 2015
Since receiving a SMARTCAP grant in August 2014 both ZetrOZ and sam® (sustained acoustic medicine), their innovative ultrasound device that enables self-administered therapeutic ultrasound therapy outside a physician’s office, have been featured on local CBS and Fox newscasts and in Triathlete Magazine. sam® also landed the number 2 spot on Medical Design Technology Magazine’s Top 10 list of intriguing new products following the Compamed trade fair in Dusseldorf.
After SMARTCAP funding, ZetrOz experienced significant growth and currently has 30 employees. The company has received several awards from the state of Connecticut in addition gubernatorial support.
ZetrOZ is now working to integrate sam® for workman’s comp insurance and auto insurance recovery strategies and have increased traction with groups that manage care in New York, New Jersey, and in Connecticut. Care managers are recommending the ultrasound therapy to speed up employee recovery and get them back to work sooner. Insurance companies have taken notice and are beginning to run analytics. ZetrOz is continuing its efforts to help educate this segment and recently held a seminar for keyplayers in the industry at Chelsea Piers in NYC.
End-user awareness and incentivizing efforts are being supported by a program called SAM Speaks, wherein users are given a significant discount on the product in return for completing an electronic survey about their experience.
Currently, ZetrOz holds one patent for OZ Inside™, the company’s ultra-low impedance ultrasound miniaturization technology, and intend to focus on obtaining broader patents in the US and abroad in the future. The company has three patents in prosecution.
According to Dr. George Lewis, the company is working to promote the FDA-cleared sam® device for chronic and acute tendonopathy as the product gains traction with professional athletes. Multiple sport teams are using the product to promote recovery including the Patriots and the Penguins and Maggie Steffens, a member of the World Champion U.S. Women’s water polo team, recently tweeted about her use of sam® to promote recovery following matches.
ZetrOz is also undergoing two clinical trials. NSBRI is supporting one of the two trials which is focused on backpain. Studies will take place in two to three different sites and the prototype for those trials is complete. Regulatory testing is being finished up now. Data collection is expected to be complete by yearend 2015. Phase one of a second trial focused on Arthritis, and supported by National Institutes of Health (NIH), is now complete as the company awaits the second phase of the trial.
The company believes that by supporting the development of the 1mHz system, NSBRI has helped open new market opportunities and is hopeful that astronauts will be allowed to test the device for further efficacy.
Oculogica, Inc.

Dr. Uzma Samadani and Robert Ritlop of Oculogica, Inc. demonstrating EyeBox-CNS, a rapid eye tracking technology that is sensitive to brain abnormalities and injuries.
Oculogica is developing EyeBoxCNS, an eye tracking technology, to detect weaknesses of the nerves that move the eye. These nerves are very sensitive to brain physiology, and thus are revealed by tracking the movement of the eyes when watching a video or reading a computer screen for 220 seconds. Oculogica believes that the technology will re-define how doctors diagnose brain injury, particularly in children. The technology can be incorporated into mobile devices for home use by everyone. In space, this technology can be used as a fitness for duty test and a measure of increased pressure on the brain, believed to cause visual impairments in some astronauts.
The technology has now been published in Journal of Neurosurgery. This ‘proof of concept’ paper demonstrates how the technology can assess the impact and location of brain swelling on the nerves that move the eyes. Read the Paper.
UPDATE: Where is Oculogica Now?
June 15, 2015
Oculogica, Inc., an early stage, neurodiagnostic company, received a SMARTCAP grant in April of 2014. The company was founded to capitalize on the discovery by researchers at the NYU Langone Cohen Veterans Center that eye tracking might provide a window to brain injuries that heretofore have been invisible to medicine’s most sophisticated imaging devices. CT scans and MRIs rarely identify concussions, regardless of their severity. But, normal eyes and the eyes of those with a brain injury do not perform the same when tested with Oculogica’s EyeBox™, an eye tracking system that is both simple and elegant. The patent pending system needs less than four minutes to detect abnormalities and identify possible causes. All patients who are conscious can be evaluated with this system. It is age agnostic, language and literacy agnostic, and, if the test is used on multiple occasions, “learning” does not impact the results.
The SMARTCAP grant enabled Oculogica to undertake a proof-of-concept trial to determine the correlation between results obtained using traditional, invasive measures of intracranial pressure with results from the EyeBox. NSBRI and NASA need a non-invasive means of detecting changes in intracranial pressure to deploy on the International Space Station to enhance their understanding of VIIP (Vision Impairment & Intracranial Pressure) Syndrome which has affected many in the astronaut corps. This trial was completed and the results have been submitted to a peer-reviewed scientific journal. The company expects that the article will be published late in 2015. The grant also enabled the company to purchase another device, allowing Oculogica to send an EyeBox to researchers at Ft. Campbell in Kentucky, where it was used to evaluate 1,000 soldiers over three weeks. The device is now being utilized in a follow-on, multi-center study to determine how to improve diagnosis of traumatic brain injuries in soldiers and veterans. The study is sponsored by the Steven and Alexandra Cohen Veterans Center and includes sites in Manhattan and Palo Alto in addition to Ft. Campbell. Eye tracking is also being performed at the Children’s Hospital of Philadelphia Concussion Center to investigate its utility for assessment of concussion patients.
Dr. Uzma Samadani, M.D., Ph.D, chief of neurosurgery for the New York Harbor Healthcare System, Co-Director of the Cohen Veterans Center for Post Traumatic Stress and Traumatic Brain Injury and co-founder of Oculogica, was an invited speaker at TedMed 2014, and presented at the 2015 annual meeting of the North American Brain Injury Society. Multiple media outlets, including Forbes, the national CBS Evening News, NBC New York and Sports Illustrated have carried stories exploring the potential impact of the technology following publication of the results of two distinct proof-of-concept studies, one in the Journal of Neurosurgery in December, 2014, and the other in the Journal of Neurotrauma in January, 2015. Three additional papers are in press.
Oculogica’s development plans include a cloud-based analytics platform that will enable the company to support a SAAS business model in addition to device sales. The company expects to have a product that is ready for the research market by midsummer 2015 and the clinical market in 2016. Oculogica has already engaged with the FDA to initiate the clearance pathway, with more than 5000 patient eye trackings to date. While the US is the initial target market for Oculogica, additional geographies are in the planning stages.
The SMARTCAP grant proved pivotal by enabling Oculogica to collect critical proof-of-concept data on the assessment of ICP, to purchase another device and hire two employees. The company has made significant progress since the grant was awarded and is now seeking to close a round of financing in the second half of 2015 that will support the company’s regulatory filing in the U.S. and market launch activities.
The Oculogica story is compelling not only for its potential utility on board the International Space Station but also for its potential on Earth to both identify brain injuries and to track recovery from those injuries. The EyeBox may allow physicians to diagnose traumatic brain injuries with far greater certainty and determine when a patient has sufficiently recovered to return to normal activities. Today, physicians must rely on highly subjective evaluations with no quantitative means to measure recovery. For the millions of athletes, trauma victims and soldiers who experience head trauma each year, the EyeBox gives their physicians a new tool with the potential to enhance diagnosis, treatment and recovery.
Pear Therapeutics, Inc.

Dr. Corey McCann demonstrates Pear Therapeutics’ e-formulations: a patient customized combination of software and medication to improve mental health.
Pear Therapeutics is developing a therapeutic platform, eFormulations, of combination therapies for a variety of brain-related disorders including pain, sleep disturbances, depression, and anxiety. A specific prescription medication is paired with an individualized software-based app that works in concert to enhance treatment outcomes. By employing the dual modality, eFormulations simultaneously impact brain experience as well as brain chemistry. Pear believes that the combination will prove to be an extremely powerful way to treat common conditions, both on Earth and in space.
UPDATE: Where is Pear Therapeutics Now?
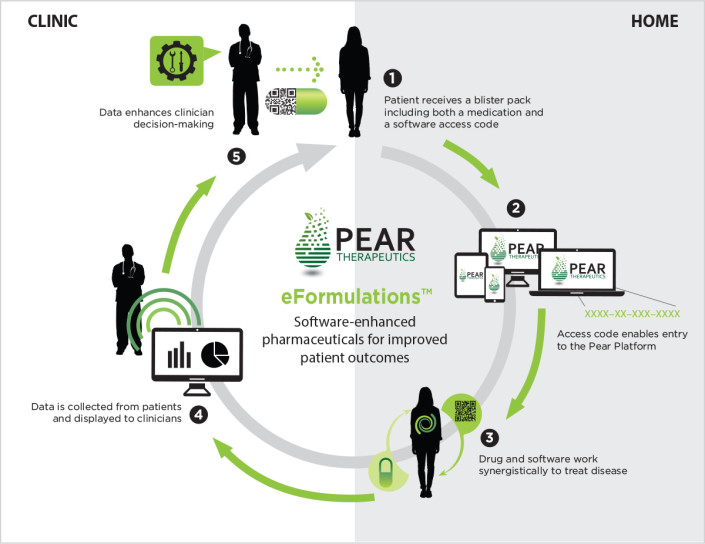
Pear Therapeutics eFormulations™ combines medication and software for synergistic clinical efforts that bridge the gap between clinical and home care
August 3, 2015
In April of 2014, Pear Therapeutics was awarded a SMARTCAP grant by the NSBRI Industry Forum Steering Council. Pear’s novel platform, eFormulations™, involves pairing a medication of proven efficacy with a digital therapy to yield enhanced therapeutic benefits. These benefits may include reducing the amount of medication needed, enhanced efficacy on approvable endpoints, and enhanced physician efficiency through analysis of the data collected by the digital therapy. Pear’s SMARTCAP grant was awarded to determine if this approach could help astronauts whose sleep is impacted by multiple day/night cycles in each 24 hour period while in space.
Pear has been working on developing the software component which will be combined with zolpidem to address insomnia. The software is drawn from a form of cognitive behavior therapy for insomnia (CBTi) and will incorporate stress reduction techniques, sleep education, and medication dosing guidance.
According to Dr. Corey McCann, CEO, the SMARTCAP grant from NSBRI enabled Pear to undertake the development of its eFormulation product for insomnia well in advance of its initial timeline and NSBRI’s introduction to experts in the field also aided in development of the product. The grant, when combined with the matching funding, has allowed Pear to go from concept to launch for this product.
In addition to combining pharmaceuticals with digital therapy, Pear intends to offer a consumer- targeted platform which will pair supplements with software. This platform will address sleep, mood, stress, memory, attention, and vision training. It will be the company’s first to market product.
The company operates two facilities, one in Boston where the pharma expertise resides and one in San Francisco with software and technology experts. The company has a team of 20 between the two locations and expects to add employees as it brings the consumer platform to market and expands its clinical and regulatory efforts for the pharmaceutical combination products.
In the first quarter of this year, Pear announced that it closed a round of financing led by 5AM Ventures, a life sciences-focused venture capital firm with offices in Menlo Park, CA & Boston, MA. This round of investment is expected to enable Pear to launch a family of supplement-software combinations, and also to develop an initial portfolio of clinical products.
Addiction is one of Pear’s primary therapeutic targets, outside the work it has undertaken with the SMARTCAP grant. An approved anti-addiction medication will be prescribed and packaged with a code unlocking a digital therapy-based software component. The physician/therapist will receive the data generated when a patient uses the software and in some cases, passive monitoring data such as vital signs, accelerometer data, and voice patterns. This data will offer a more complete picture of the patient’s status and progress enhancing the physician’s ability to refine the treatment algorithm. Additional therapeutic targets are schizophrenia, post-traumatic stress disorder (PTSD), and general anxiety.






