Long-duration spaceflight poses many health risks for human explorers. Astronauts in microgravity are susceptible to developing weaker bones, which is also a serious problem on Earth for those who suffer from osteoporosis and other bone diseases. NSBRI Postdoctoral Fellow Dr. Stephanie M. Carleton has developed a research project to study how the absence of myostatin — a negative regulator of muscle growth — impacts the bone quality of mice with brittle bones, which models the segment of the human population with osteoporosis. Carleton’s hypothesis is that the muscle mass in the mice with the mutation and osteoporosis-like symptoms will increase during exercise, leading to stronger bones due to the added stress to the bones from the larger muscles. This research could lead to effective bone loss countermeasures during long-duration spaceflight and improved treatments for patients suffering from bone loss.
Overview
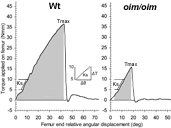
Representative graphs showing femur torque versus angular displacement for wildtype and oim/oim femora at four months of age. Torsional ultimate strength (Tmax) is the maximum applied torque required to fracture the bone. Energy to failure (U; shaded area) is the amount of strain energy the bone can absorb prior to fracture. The insert illustrates a least-squares fit line to data from 5 to 10 Nmm with the torsional stuffness (Ks) derived from the slope of this line. Image courtesy of Stephanie Carleton, Ph.D. Click here for larger image.
A Combinatorial Approach of Exercise and Myostatin Inhibition to Enhance Compromised Bone (Postdoctoral Fellowship)

Exercise studies using mice with osteogenesis imperfecta (the brittle bone disease) could help humans who have the disease and astronauts who lose bone density on space missions. Researchers hope to learn whether exercise, such as walking on a treadmill, will improve bone density and strength in this mouse and others. Photo by Shane Epping, Mizzou Wire at the University of Missouri. Click here for larger image.
Principal Investigator:
Stephanie M. Carleton, Ph.D.
Organization:
University of Missouri-Columbia
Technical Summary
Bone is inherently mechanosensitive, responding and adapting to its mechanical environment. Bone formation occurs in response to high mechanical loads, often changing its geometry to strengthen the skeleton. The largest physiological loads bones typically experience are from muscles with bone strength directly proportional to muscle mass.
Myostatin (mstn) is a member of the transforming growth factor- (TGF-) super family and is a negative regulator of skeletal muscle growth. When the myostatin protein is missing or non-functional, the result is increased muscle growth with a concomitant increase in bone strength. Recently a completely myostatin-deficient child was described whose quadriceps muscles were 7.2 standard deviations above normal but without any detrimental health consequences. Myostatin-deficient mice have a similar phenotype: increased muscle mass and bone strength compared to Wt mice. Previous studies demonstrated that mice completely deficient for myostatin (mstn/mstn) have a 40 percent increase in quadriceps muscle mass, an 11 percent increase in bone mineral density and a 20 percent increase in radial ultimate force relative to Wt mice. When mstn/mstn mice were subjected to treadmill exercise, radial ultimate force improved by an additional 17 percent. Exercise has also been shown to be beneficial for human patients at an increased risk for fracture (i.e., osteoporosis, osteogenesis imperfecta).
Based on these previous reports, this project was designed to determine if a combination of exercise and myostatin haploinsufficiency could improve the bone strength in mice with compromised bone (+/oim and G610C OI).
Specific Aims
- Determine if +/oim and G610C OI mice are able to tolerate and respond to treadmill exercise.
- Determine if myostatin haploinsufficiency will ameliorate the bone phenotype of +/oim and G610C OI mice.
- Determine if a combination of exercise coupled with myostatin haploinsufficiency will increase muscle mass and bone strength in +/oim and G610C OI mice.
Preliminary data demonstrates the reduction in bone strength seen in female +/oim animals was partially rescued when those mice either underwent treadmill exercise or were also haploinsufficient for the myostatin protein (+/mstn). Treadmill exercise did not appear to impact femoral geometry. However, modest improvements were seen in femoral torsional ultimate strength and energy to failure in both Wt and +/oim mice, though these improvements were not significant. Treadmill exercise did significantly reduce whole bone and material stiffness in both Wt and +/oim mice. Curiously, treadmill exercise did not appear to be beneficial for male Wt or +/oim mice. Myostatin haploinsufficiency marginally improved femoral torsional ultimate strength and energy to failure in +/oim mice, though not significantly. Myostatin haploinsufficiency did significantly reduce whole bone and material stiffness in +/oim mice. Taken together, this data indicates that both treadmill exercise and myostatin deficiency has the potential to improve bone strength and reduce stiffness in +/oim mice with compromised bone.
In order to complete this study, additional female +/oim mice will be evaluated to assess the impact of myostatin haploinsufficiency on both muscle size and bone strength and stiffness. Additionally, the same studies will be performed on male +/oim mice to determine if a gender difference exists in the ability of the bone to respond to myostatin haploinsufficiency. Male and female G610C OI mice will also be evaluated for their ability to respond to treadmill exercise. The potential impact of maternal myostatin haploinsufficiency on the muscle size and bone strength of the offspring will also be evaluated. Finally, +/oim mice of both genders that are also haploinsufficient for myostatin will be subjected to treadmill exercise to determine if exercise coupled with myostatin haploinsufficiency further improves bone strength in +/oim mice.





