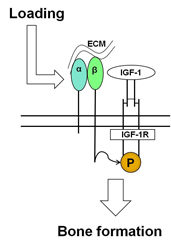
Bone loss is a serious health risk of living in the reduced gravity of space for long periods of time. In space, bones no longer experience the stimulation, or loading, that occurs during activities such as walking and running in normal gravity. Scientists do not fully understand the mechanisms that cause this bone loss. Research indicates that insulin-like growth factor type 1 (IGF-1), a hormone that plays a role in childhood growth and in tissue building in adults, is ivolved in bone formation after load-bearing activities such as walking. When weight-bearing activity is removed, IGF-1 fails to increase bone formation.
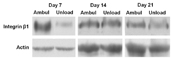
Dr. Kubota’s project seeks to understand what role the IGF-1 receptor (IGF-1R) and beta 1 and beta 3 integrins (which help cells attach to tissues) play in the formation of bone following load-bearing activity. The study will examine changes in mice lacking IGF-1R and beta 1 and 3 integrins when the mice are placed in conditions simulating reduced gravity followed by normal gravity. The project seeks to learn more about the role of IGF-1 and beta 1 and 3 integrins in spaceflight-induced bone loss.