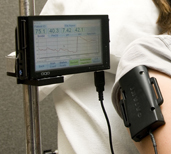
NASA is designing new spacesuits to meet the needs of future space exploration. Real-time measurement of metabolic rate during astronaut activity is a key function of the biosensor suite which is planned for the new spacesuit. Through her previous NSBRI project, Dr. Babs R. Soller and colleagues developed a biosensor that uses near infrared spectroscopy (NIRS) to noninvasively measure blood and tissue chemistry important in assessing metabolism.
With this project, Dr. Soller is working on new algorithms for the biosensor, enabling it to accurately and continuously assess metabolic rate. Dr. Soller’s team is also working on a smaller version of the sensor so that it will fit comfortably within the spacesuit. The sensor and algorithms are intended for use during the development of new spacesuits and ultimately when astronauts undertake future exploration missions. Earth applications include noninvasive, real-time assessment of metabolic rate as part of a fitness or weight loss program.