Blood analysis is one of the best tools for monitoring astronaut health and physiologic changes due to microgravity and radiation exposure. Dr. Yu-Chong Tai and colleagues are developing a light-weight and portable blood-analysis instrument using a lab-on-a-chip technology to perform blood analyses in space. The form factor of the instrument is chosen to be suitable for a medical kit aboard the International Space Station, or even Crew Exploration Vehicle. The instrument can evaluate, count and identify different types of blood cells, and analyze serum and plasma protein biomarkers. In addition to health monitoring, the technology can also be used to quickly determine the effectiveness of countermeasures deployed during flight.
Overview
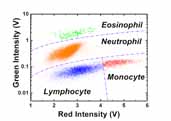
Results showing the 4-part white blood cell differential count. Click here for larger image.
In-Flight Blood Analysis Technology for Astronaut Health Monitoring

The blood count in-a-box prototype and the disposable microfluidic cartridge. Click here for larger image.
Principal Investigator:
Yu-Chong Tai, Ph.D.
Organization:
California Institute of Technology
Technical Summary
This project is a continuation of a related project entitled "Handheld Body-Fluid Analysis System for Astronaut Health Monitoring," in which we explored electrical impedance sensing, fluorescence optical sensing and flow separation of blood cells in microfluidic devices and portable platforms. We successfully demonstrated fluorescent sensing and counting for white blood cells (WBC) and two-part differential with a portable prototype micro flow cytometer.
For this project, the major efforts are to extend the two-part WBC differential to a five-part WBC differential, add cell surface marker detection and analysis capability to the platform repertoire, and add plasma protein detection and analysis capability to the platform repertoire.
Specific Aims
- Extend prototype to a five-part WBC differential.
- Add analysis of WBC subtypes (e.g., CD4+ T helper and natural killer cells).
- Add serum/plasma protein biomarker analysis (e.g., for infection, radiation and bone loss monitoring).
Our approach to achieve the objectives is to extend the capability of the micro flow cytometer to enable a more comprehensive WBC differential and allow detection of fluorescent labels attached to ligands used for cell surface marker and plasma protein detection. The second component necessary for extending the platform capability is the offline data analysis software. This software is being developed in Matlab to facilitate both quantitative assessment of fluorescence detection, cell/analyte recognition and quantitation.
In the last year, we searched for a new staining method and optimized the previously proposed acridine orange staining. We successfully developed a four-part differential assay (i.e., lymphocyte, monocyte, neutrophil and eosinophil) with a cocktail staining of fluorescent dyes: fluorescein isothiocyanate (FITC) and propidium iodide (PI). The differential assay was investigated in a correlation study with the commercial hematology analyzer and further verified with the purified WBC types. For the acridine orange assay, the differential capability was also extended from three-part (lymphocyte, monocyte and neutrophil) into four-part (lymphocyte, monocyte, neutrophil and eosinophil). The time and temperature dependence of the acridine orange staining are also being investigated.
We also worked on improving the proposed platform's detection. Two different approaches, including a commercial mini-spectrometer and an eight-color fluorescence reader with an eight-channel photomultiplier tube module, have been implemented. Single-cell fluorescence emission spectrum has been measured on the mini-spectrometer prototype. The information about the subtle differences of the spectrum can be used to optimize the wavelength range of the multi-color measurement for capturing these differences. Multicolor fluorescent beads have been successfully measured on the eight-color reader.
Those two approaches are investigated in order to extract as much useful information as possible from the emitted signal. The additional spectral information should provide better discrimination between multiple fluorophores used simultaneously. It may also provide additional information about the intracellular environment in which acridine orange fluorescence occurs, leading to efficient WBC subtype discrimination.
To analyze WBC subtypes, we initiated the development of synthesized peptides, which can be utilized to selectively target and bind to leukocytes. The binding peptides were custom synthesized with a fluorescein fluorophore attached to their N-terminus for binding quantification. Seventy-two potential peptide candidates have been tested using a modified protocol for leukocytes utilized in our studies. Several peptide binding assays have been performed to screen for potential peptides that will preferentially bind to leukocytes. Among the results, four peptides from our initial library exhibited a two-to-three times higher binding strength to the B-cells compared to the other peptides but showed minimal preference compared with the control group in our primary tests. We are in the process of running additional peptide binding assays using a new peptide library against cultured cell lines (B- and T-cells).
Plans for the coming year
We will work on the prototype with the spectrum analysis capability and the one with the eight-color reader for developing the staining assay. Ability to discriminate among multiple color emissions will provide the capability to detect multiple ligands simultaneously and may help in performing a five-part WBC differential with a single stain, such as acridine orange.
Further, we will continue to work on developing the staining assay for the test platform, expanding the differential capability of the staining assays to five-part (lymphocyte, monocyte, neutrophil, eosinophil and basophil). A staining assay with a dye cocktail of PI, FITC and Basic Orange 21 is currently being investigated. For the WBC subtype differential with synthetic peptides, we are running additional peptide binding assays using a new peptide library. These assays will be run using cultured cell lines (B- and T-cells), and additional leukocyte subtypes will also be tested when promising peptide candidates are identified.





